Back to Block 5
Back to Block 4
Back to Block 3
Back to Block 2
Back to Block 1
Back to S103 | Block 6: Our world and its atoms. Contents
Doing chemistry
The Lyme volcano: iron pyrite
On the Bolivian frontier: sodium nitrate
The vanishing lake: sodium chloride
A first look at acids
Doing chemistry quantitatively
Does the total mass change in a chemical reaction?
Copper and copper oxide
Copper oxide from copper and nitric acid
The concept of chemical composition
Elements and compounds
A first look at chemical symbols
A first look at chemical names
The oxides of carbon
The composition of carbon dioxide
A new oxide of carbon
An atomic theory
Elements, compounds and chemical symbols: a second look
A first look at chemical formulae
A first look at chemical equations
Methane and a burning question
Getting to know gases
Pressure
Atmospheric pressure
Pressure and gas volume: Boyle's law
Temperature and gas volume: Charles' law
Revisiting the particle model of a gas
The densities of gases
The eagle in the dung heap: ammonium chloride and ammonia
Gay-Lussac's law
The Italian job
Avogadro's hypothesis
The Karlsruhe conference
Cannizzaro's principle
Revisiting equation-balancing
The flame of burning hydrogen
The flame of natural gas
Relative atomic masses
Relative molecular masses
The concept of the mole
Using moles to calculate amounts of reactants and products
Moles in space
Finding chemical formulae from chemical compositions
Empirical and molecular formulae
A first look at valency
A second look at acids
Salts
Basic hydroxides and alkaline solutions
Neutralization
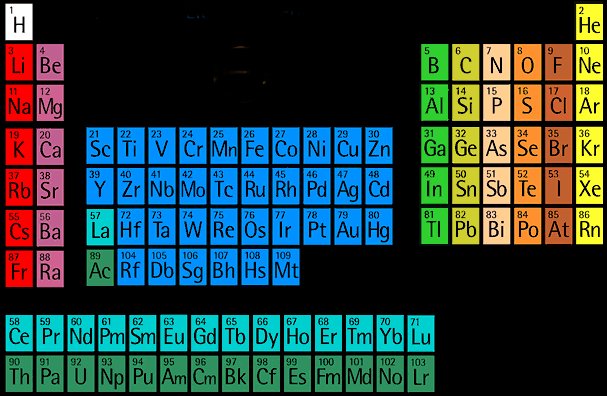
Chemical periodicity
The noble gases
The alkali metals
Atoms and electrons
A world of charge
Ions
Balanced equations containing ions
A final visit to acids and basic hydroxides
Taking the atom apart
Radioactivity
Radium
Inot the heart of the atom
Into the atomic nucleus
Isotopes
How big is a zillion?
How small is an atom?
|