S103 Discovering Science Diary
Block 5
[ Block 1 ] [ Block 2 ] [ Block 3 ] [ Block 4 ] [ Block 6 ]
2nd March
I have finished the block (hurrah!) and done the TMA question roughly. I understood it all better as I went on and it came together at the end not too badly, however, time to move on ........ (phew!)
 Arrggghhhhhh! Arrggghhhhhh!
24th February
I am quite baffled by this! So, I'm going to put some salient points onto here, as I get to them. Perhaps I'll put them all together at the end of the block and maybe they might make some sense??
Examples of energy transfers and conversions

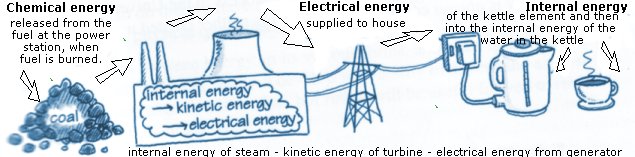
The law of conservation of energy:
In any process, the total amount of energy is always constant.
Energy cannot be created or destroyed.
(From Block 5, Section 3 summary)
The work W done by a force on an object is equal to the magnitude F of the force multiplied by the distance d that the object moves in the direction of the force while the force is acting on it.
W = Fd
The work done on an object is equal to the energy transferred to that object.
The SI unit of energy is the joule
1 J = 1 N x 1 m = 1 N m = 1 kg m2 s-2
(eg If F = 415 N and d = 15m,
Fd = 415 N x 15 m
= 6.225 x 103 N m
= 6.2 x 103 N m (to two sig figs)
So, W = 6.2 x 103 J (as 1 J = 1 N))
If an object with mass m is moving with speed v, then its energy of motion, known as the kinetic energy Ek, is given by
Ek = ½ mv2
Example:
If m (mass) is 800 kg and v (velocity) is 20 m s-1,
Ek = ½ x (800 kg) x (20 ms-1)2
= 400 kg x 400 m2 s-1 - 1.6 x 105 kg m2 s-2
= 1.6 x 105 J
So, Ek (the kinetic energy) = 1.6 x 105 J
Power P is the rate at which energy E is converted or tranferred
P = E/t
The SI unit of power is the watt.
1 W = 1 J s-1
Example
If 0.6 J of energy is transferred in 0.2 seconds,
the P = E/t
= 0.6 J/0.2 s
= 3 J s-1
= 3 W
a ∝ (is proportional to) b and a ∝ c, then a ∝ bc. Or a = kbc, where k is the constant proportionality.
Motion under gravity
The magnitude of gravitational force between two objects, increases when the masses of the objects increases and decreased as their separation increases. Gravitational force experienced by an object is proportional to its mass.
Fg is proportional to m
The acceleration due to gravity experienced by any object that is fallin gfreely close to the Earth's surface is a constant. It has the same value, irrespective of the mass of the object.
Value of gravitational acceleration (g) near to the Earth's surface = 9.8 m s-2 (near the moon is roughly 1.6 m s-2
Fg = weight of the object.
Fg = mg or weight = mg
In order to lift an object, we have to apply an upward force mg to overcome the downward force of gravity.
W = Fd = mg x h = mgh
example, the work done in lifting a 12 kg suitcase from floor level up to a luggage rach 2.0m above the floor:
W = mgh
W = 12 kg x 9.8 m s-2
= 235.2 kg m2 s-2
= 240 J
Gravitational (potential) energy = Eg
Change in gravitational energy = mg x change in height
Δ Eg = mgΔh
Potential Energy - stored energy
If gravity is the only force acting on an object, the sum of kinetic energy and gravitational energy is constant. Increases in kinetic energy are balanced by decreases in gravitational energy, and vice versa.
Total internal energy of an object is the sum of kinetic and potential energies of all of the molecules in the object.
Heat is energy which flows from a higher temperature to a lower temperature because of the temperature difference, and when heat is transferred to an object, the internal energy of that object increases.
q (heat) is proportional to ΔT
c = constant of proportionality or specific heat
c = q/mΔT (specific heat = heat divided by mass times change in temperature.
Absolute temperature
Introduced by Lord Kelvin, to define absolute zero.
-273.15 degrees C = absolute zero
O K (Kelvin) = absolute zero
Therefore, the kelvin scale is always 273.15 degrees above celsius scale (although the size of a kelvin is exactly the same as the size of a celsius degree).
The latent heat of vaporization (L), SI unit = J kg-1
q = Lm or L = q/m (q measured in J)
Example, the energy required to evaporate 5.1 x 1017 kg (mass) of water from the Earth's surface (assuming that the latent heat of vaporization of water is 2.6 x 106 J kg-1)
= (5.1 x 1017) kg x (2.6 x 106) K = 1.3 x 1024 J
Electrical Energy
Charged objects with the same sign (negative or positive) repel
Charged objects with opposite signs attract.
Equal amounts of positive and negative charge = neutral
Electrons are free to move in electrical conductors (example metals and salty water) but not able to move freely in electrical insulators (eg rubber, glass, most plastics).
SI unit of charge is the coulomb, C. Electric current = I
I = Q/t (Q = electric charge). SI unit of current = 1 A (amp) = 1 C s-1
The change in electrical energy when charge Q flows through a voltage difference ΔV is
ΔE;e = QΔV. The SI unit of voltage difference is the volt V.
Electrical power is the rate at which electrical energy is converted into other forms of energy
P = Δ Ee/t = 1ΔV
Examples
An electric kettle, operating from the 240 V mains electricity supply with a heating element current of 8.4 A, has a power rating of
8.4 A x 240 V = 2016 AV or 2016 W or 2.0 kW.
The Sun's Energy (Nuclear Fusion of hydrogen into helium)
Electromagnetic radiation from the Sun is the ultimate source of energy for life on Earth.
The energy equivalent to mass m is given by Einstein's equation:
E = mc2
where E = amount of energy equivalent to mass, where c is the speed of light (3.0 x 108 m s-1)
For example, the amount of energy equivalent to 1 kg of matter =
E = 1 kg x (3.0 x 108 m s-1)2 = 9 x 1016 kg m2s-2 = 9 x 1016 J.
If the energy of an object increases, then its mass also increases.
| Quantity | Symbol | Unit |
| heat | q | joule (J) |
| electric charge | Q | coulomb (C) |
| energy | E | joule (J) |
| weight | Fg | newton (N) |
| power | P | watt (W) |
| work | W | joule (J) |
| force | F | newton (N) |
| voltage difference | ΔV | volt (V) |
| electric current | I | amp (A) |
| temperature | T | kelvin (K) |
| SI unit | Equiv unit |
| I = Q/t | A | C s-1 |
| W = Fd | J | N m |
| P = E/t | W | J s-1 |
| F = ma | N | kg m s-2 |
| ΔEe = QΔV | J | C V |

Back to Block 4
Back to Block 3
Back to Block 2
Back to Block 1
Back to S103 | Block 5: Energy. Contents
Energy conversion and conservation
Work, energy and power
Force and work
Kinetic energy
Power
Motion under gravity
Work done by gravity
Work done against gravity - gravitational potential energy
Gravitational energy and energy conservation
Other forms of potential energy
Energy in biological system
Internal energy
What is internal energy
Specific heat - relating heat transfer to temperature change
The absolute scale of temperature
Latent heat of vaporization
Electrical energy
Electric charge
Conductors and insulators
Electric current
Electrical energy and voltage
Electric power
Energy from the Sun
Uses of solar energy
The Earth's GMST - a final calculation
Nuclear fusion - energy from the heart of the Sun
|
|
Arrggghhhhhh!


